The global asthma treatment market size was reached at USD 27.32 billion in 2024 and is expected to be worth around USD 41.02 billion by 2034, growing at a compound annual growth rate (CAGR) of 5.9% over the forecast period from 2025 to 2034. The asthma treatment market will grow at a significant rate owing to increasing prevalence of respiratory disorders, rising pollution levels, and advancements in biologics and inhalation therapies. Additionally, improved awareness, early diagnosis, and government initiatives to enhance healthcare infrastructure are driving demand for innovative and personalized asthma management solutions.
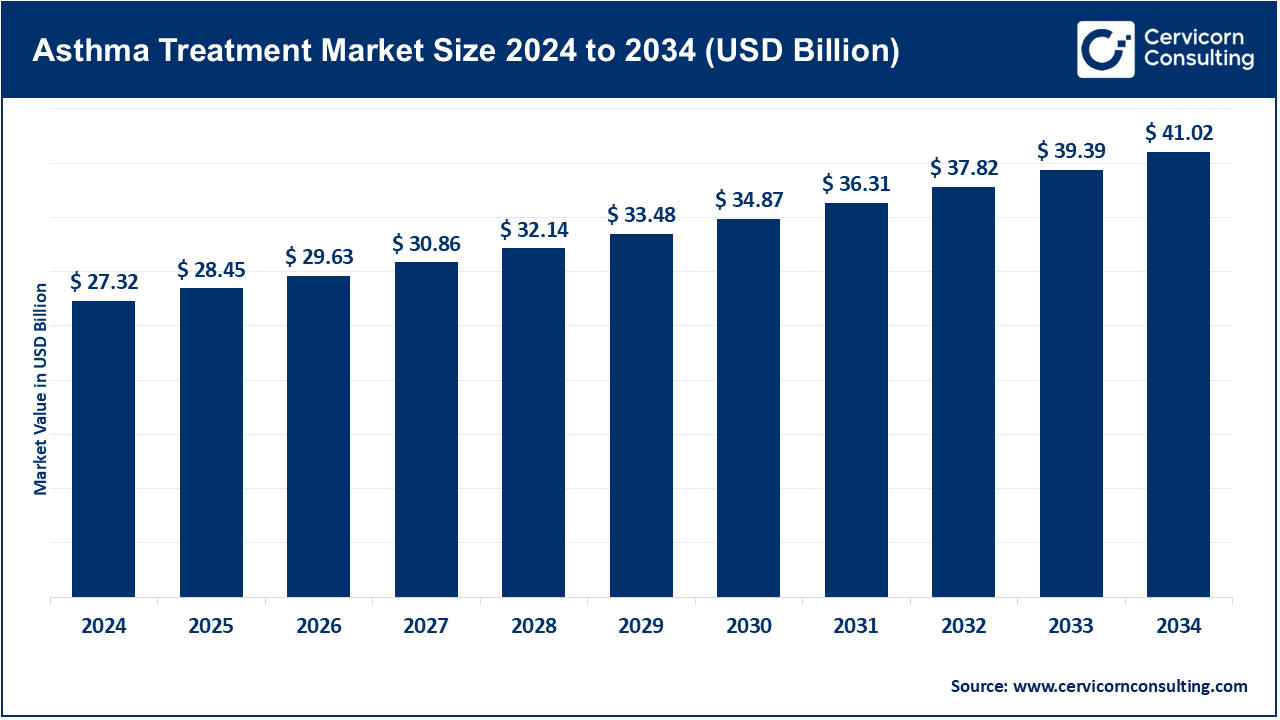
The prevalence of asthma, as well as pollution levels, is directly contributing to the rapid growth of the asthma treatment market. The market continues to expand due to advancements in personalized medicine as well as new innovations such as biologics, smart inhalers, and AI-powered monitoring tools. These advancements increase adherence to treatment protocols and precision in treatment execution. As pharmaceutical companies work on more precise therapeutics for complex asthma, symptom monitoring is being automated digitally in real time. Integration of technology with health care is developing advanced systems for the delivery of care. Focused innovation stemming from policies for expedited approval is being amplified. All in all, the shift is in the direction of more responsive, nimble, sophisticated systems for asthma care that prioritize the patient.
Report Scope
| Area of Focus | Details |
| Market Size in 2025 | USD 28.45 Billion |
| Expected Market Size in 2034 | USD 41.02 Billion |
| Projected Market CAGR 2025 to 2034 | 5.90% |
| Top-ranking Region | North America |
| Top Expanding Region | Asia-Pacific |
| Key Segments | Treatment, Route of Administration, Patient Type, Technology Integration, Distribution Channel, Region |
| Key Companies | AstraZeneca, Teva Pharmaceutical Industries Ltd., GlaxoSmithKline plc, Boehringer Ingelheim International GmbH, Roche Holding AG / Novartis AG, Merck & Co., Inc., Koninklijke Philips N.V., Sanofi-Aventis SA, MundiPharma. |
Long-Term Control Medications: These are daily treatments, usually inhaled corticosteroids (ICS) and long-acting bronchodilators, aimed at reducing airway inflammation and preventing exacerbations of asthma. The WHO reports that as of 2023, only about 50% of low or middle-income countries, often referred to as lower-income countries, regularly stock essential ICS medications. These health systems have restricted the procurement and distribution of long-term agents to combat under-treatment through 2024. Tezepelumab and dupilumab injections—now positioned alongside ICS for severe cases—were approved in 2022 and 2023. The FDA endorsed tezepelumab for home-administered biologic control in early 2025. These policies illustrate the worldwide focus on integrating proactive and preventive strategies in the management of asthma.
Asthma Treatment Market Revenue Share, By Treatment, 2024 (%)
| Treatment | Revenue Share, 2024 (%) |
| Long-Term Control Medications | 64% |
| Quick-Relief Medications | 36% |
Quick-relief Medications: SABAs are classified as Short-Acting Bronchodilators which are a type of quick-relief medication. During an acute asthmatic episode, bronchospasm responds to SABAs quickly and effectively. As reported by the World Health Organization in 2024, approximately 70% of primary care clinics in underserved countries have SABAs available. Despite this, dependable access to these medications remains a challenge. Recent WHO asthma surveys indicate a disproportionate reliance on rescue inhalers, emphasizing short-term relief rather than long-term prevention. Patient education aimed at the overuse of rescue devices has been mandated as of mid-2024 in many national guidelines. An exacerbation of environmental triggers from 2023-2025 has heightened the need for quick-relief medication management. Subsidized inhalers are now being distributed to at-risk populations during high-pollution seasons. These policies strive to balance urgent responsiveness with sustainable control.
Injectables: The Injectable route encompasses biologics given subcutaneously or IV for severe asthma. Tezepelumab's home administration approval in early 2023 marked a milestone for non-hospital biologic administration. According to mid-2024 data from the CHRONICLE registry, 67% of severe-asthma biologic starts were with injectables, including dupilumab and tezepelumab. The mid-2024 expansion of labeled use added nasal polyps and COPD overlap. In 2025, insurers reimbursed home-administered injectables based on outcomes-based contracts. This route offers unparalleled convenience and accessibility for severe asthma therapy.
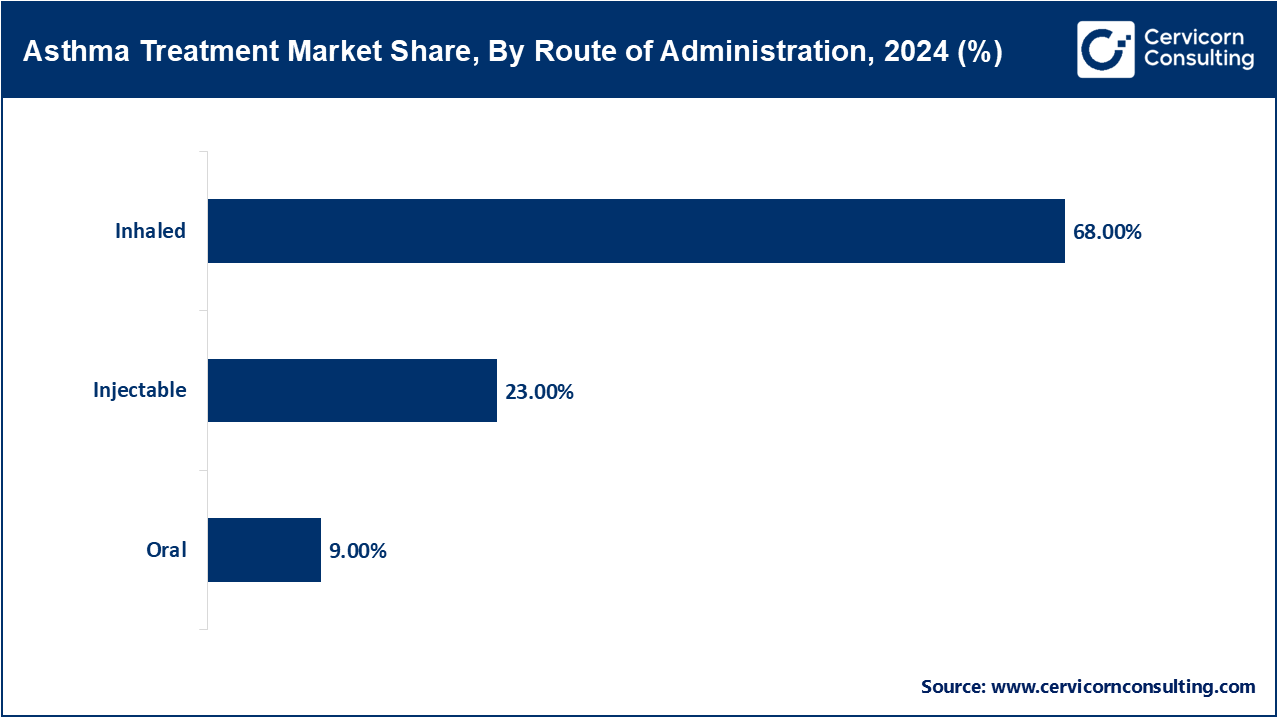
Oral: In the case of asthma which is moderate to severe, it can be managed with leukotriene modifiers and system corticosteroids. In the year 2023, WHO stated that oral controllers were included in approximately 20% of asthma management plans within LMICs. Justifications for prescribing oral montelukast for long-term control were confirmed with the updates to Essential Medicines List in late 2024, however, there are still concerns regarding the systemic steroid pills and the potential for side effects, over-prescription, and a lack of justified clinical uses. As of early 2025, there was a shift in WHO guidance towards reducing the use of oral steroids and favoring inhaled or biologic therapies. These policy changes seem to strive to provide an adequate therapeutic benefit while minimizing the risk of adverse events.
Inhaled: The Intermittent reliever and Combination Inhaler ICS, LABA are the most frequently utilized and therefore, the most served routes. As stated ICS is available in over 80% of pharmacies in high- and middle-income countries as per the 2023 WHO report. In 2024, WHO encouraged the adoption of inhaler devices without propellants to reduce carbon emissions. Additionally, in 2025 there was an announcement about a partnership between a major inhaler manufacturer and several international organizations for disease control to provide subsidized inhalers to rural regions in Africa. In February 2024, select clinics in Europe initiated the piloting of digital-dose counter inhalers, which enhance monitoring adherence. The basic and advanced levels of asthma care still primarily utilize inhalation as the method of delivery.
Hospital Pharmacies: Biologics and sophisticated therapies are increasingly important to modern hospital pharmacy practice. According to WHO, as of 2024 more than 90% of hospital pharmacies in high-income countries stock asthma biologics. Tezepelumab, which was approved for hospital use in 2023 and will be authorized for home use in 2025, demonstrates the importance of this channel. In late 2024, pharmacies in several regions began to incorporate smart inhaler monitoring systems into their standard operating procedures. Those hospitals are now training and supervising complex dose and education systems for advanced asthma therapies. Thus, hospital pharmacies function as both logistics and teaching centers for asthma care.
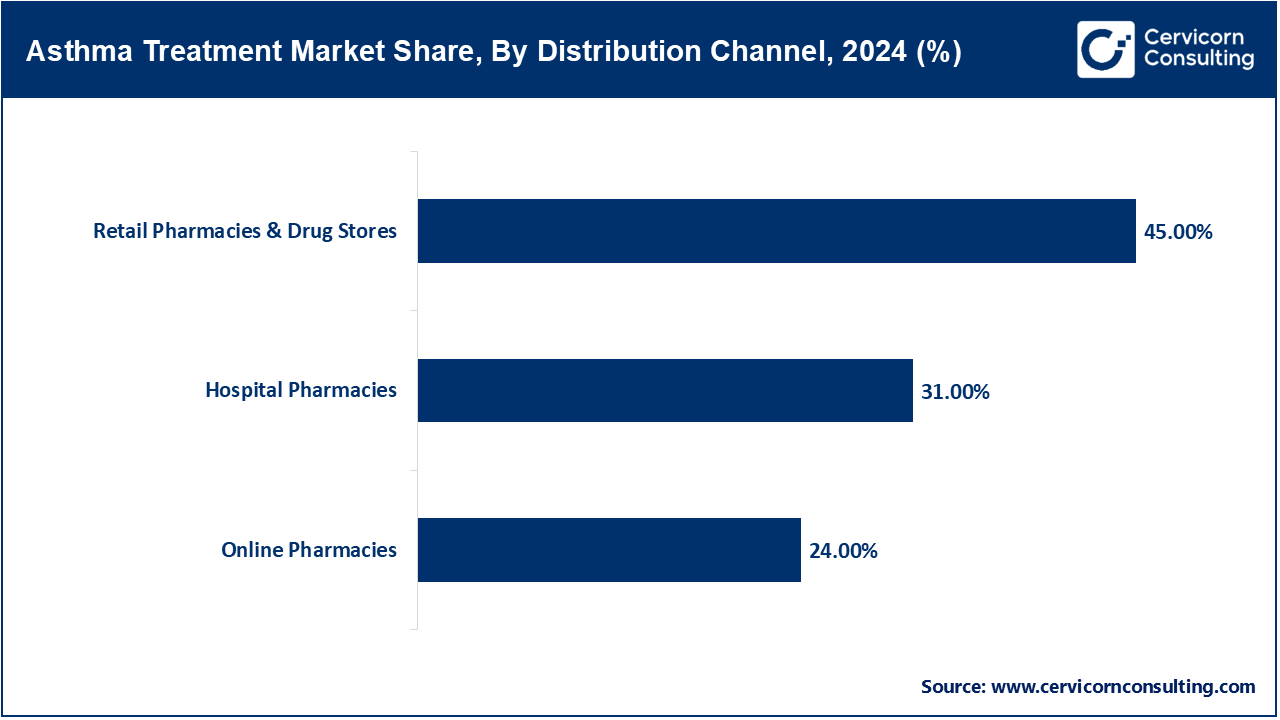
Retail Pharmacies & Drug Stores: Retail pharmacies are the first point of contact for both OTC and prescription inhalers and oral medications. WHO 2024 estimates suggest 75% of retail pharmacies located in urban areas have ICS/LABA combo inhalers. Early in 2025, a national drug code change enabled the tracking of smart inhalers at the retail level. Many retail chains in Europe and North America started offering inhaler technique teaching in 2023. Collaboration between retailers and digital health companies to embed inhaler-tracker apps into inhalers is on the rise. This area continues to be important for patient education, access, and proactive management.
E-Pharmacies: E-pharmacies sell asthma medication through e-commerce platforms, which increases convenience and potentially access. Since 2023, the WHO has been tracking the quality of online listings for OTC medications, finding that approximately 10% of listed digital products for asthma lacked proper verification. Laws passed during 2024-2025 regulated online sales to require confirmation of prescription, validation, and authenticity of the products being sold. During the coronavirus period, there was a 40% increase in the sales of refill and OTC medications and most of those gains have remained. As of 2024, telehealth services have partnered with licensed pharmacies enabling integrated prescribing and delivery. The online channels are widening the range, especially for the rural elderly and people with physical disabilities.
Pediatric patients aged 0 to 17 years: Pediatric asthma describes children with persistent inflammation of the airways accompanied by wheezing and dyspnoea. Asthma affects about 300 million people globally in 2023 with children accounting for a significant portion; the WHO approximates 0.7 per 100,000 pediatric deaths annually. In rural Karnataka, India, around 4% of children are reported to have current wheezing, with 63.9% of those cases being classified as severe, which indicates a major therapeutic gap illustrating slack. Schools in Africa report ≤33% formally diagnosed. From 2023-2025, pilot programs for digital monitoring apps and smart inhalers have been conducted in children. In the educational environment, innovations such as portable wheeze detectors are being tested. These attempts illustrate the persistent stagnation in the timely diagnosis within the context of prompt multi-dimensional frameworks, adherence scaffolds, medication distribution systems, and proactive medication frameworks addressing system gaps.
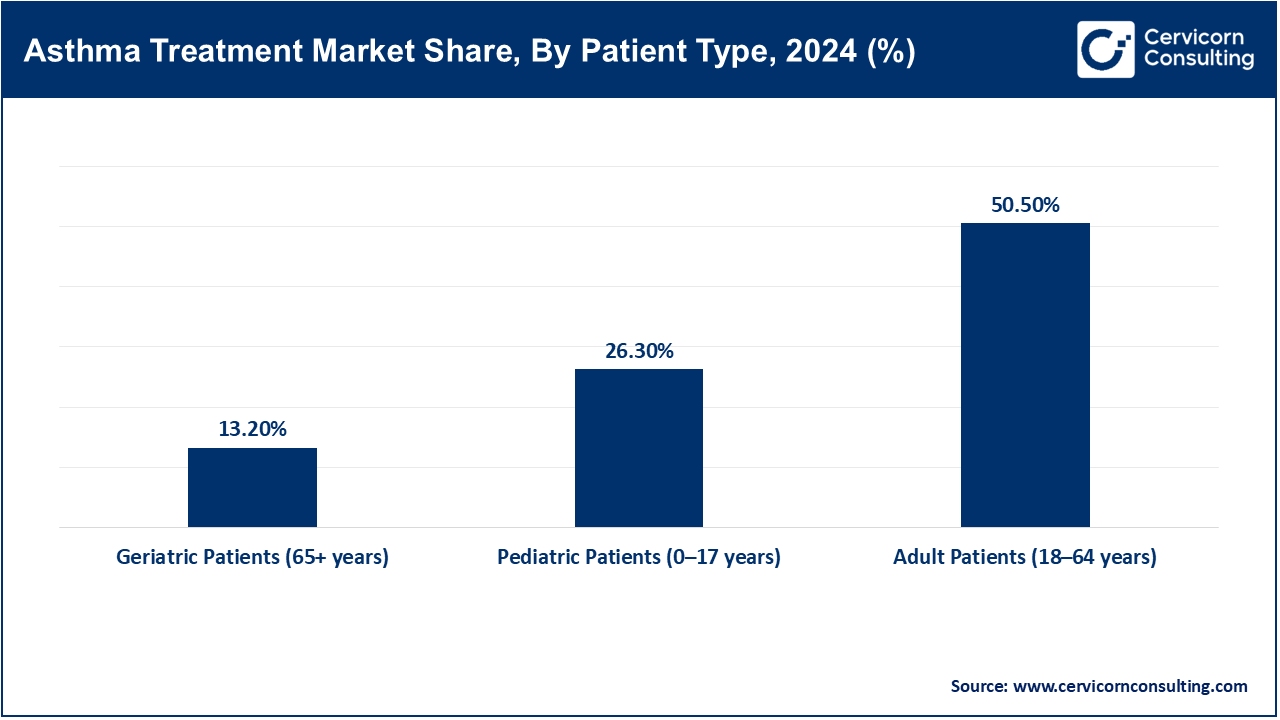
Adult Patients (18–64 Years): This cohort includes working adults in the chronic stage of the disease. In 2021, the US recorded over 20 million adults with asthma, resulting in $94,000 worth of hospital admissions and nearly 1 million ER visits each year. Relief medications and digital inhalers that sync with smartphones are more commonly used during this stage. In 2023, the AAAAI and CDC authorized remote monitoring codes for smart inhaler and mobile spirometer users. Per Baystate Noble Hospital pilot studies, AI-enabled RPM apps reduced ER visits by thousands between 2023 and 2025. These systems strengthen clinician supervision and enhance chronic adherence. The adult asthma strategy pivoted to focusing and refining chronic illness management with sophisticated analytics.
Geriatric Patients (65+ years): Asthmatic older adults frequently experience reduced lung function alongside other chronic diseases and polypharmacy. In 2023, Medicare RPM coverage extended to over 16,000 patients, improving medication adherence and reducing hospitalizations. Pulse oximetry devices which allow self-monitoring of breathing and were approved in 2023, assist geriatric patients. Remote patient monitoring (RPM) systems put in place during the pandemic continued through 2025, enabling physicians to monitor respiratory metrics during non-contact visits. The acceptance of Remote Patient Monitoring (RPM) is driven by the newly created billing codes for RPM services from Medicare. The outcome of RPM is particularly beneficial for older patients. These changes demonstrate the evolution of policy regarding patients with asthma in the geriatric population towards promoting self-sufficiency and lessening hospital admissions.
AI & Bluetooth Integrated Devices: Smart inhalers equipped with Bluetooth and artificial intelligence technologies monitor patient adherence to inhalation and medication protocols in real time. They improve adherence by notifying patients and caregivers, thus minimizing hospital admissions. ProAir Digihaler by Teva was integrated into remote asthma management systems in U.S. hospitals in 20203, which operated on a data driven system. An NIH study showed users of smart inhalers reported over 30% reduction in monitored ER visits. Other systems, like NHS in the UK, are also pilot testing these technologies. The initiative was further extended in 2024 with digital inhalers from Propeller Health and AstraZeneca. By 2025, smart inhalers are anticipated to be incorporated into a fully integrated chronic precision medicine framework.
Connected Nebulizers: Connected nebulizers are portable, internet-connected devices for aerosol delivery systems, which stream data about use and techniques to the prescribing physician in real time. The continuous use of these devices during the pandemic has supported the management of chronic asthma, including telehealth supported rural clinic connected nebulizer programs Philips launched in India in 2023. According to ATS in its 2024 whitepaper, connected nebulizers are expected to reduce the tendency to over-prescribe steroids in pediatric asthma patients. Connected nebulizers come with automated caregiver alerts and reminder systems. Clinicians are able to view real-time dashboards providing data to make proactive changes. In 2025, some Medicaid programs in underserved areas began reimbursing connected nebulizer therapy. These advances improve clinical responsibility and access in the management of severe asthma.
Digital Adherence Monitors: Digital adherence monitors are small electronic sensors embedded into or affixed on the inhalers which track the temporal aspects of doses taken by the patient. Through mobile apps, data is uploaded and processed which track specific behaviors by the clinician and enables proactive intervention where necessary. The U.S. Department of Health and Human Services published a study in 2023 stating that patients with adherence monitors showed 26% improvement in regular asthma medication use. The Centers for Medicare & Medicaid Services (CMS) has recently issued reimbursement codes as of 2024 covering remote therapeutic monitoring (RTM) of adherence. Major firms like Cohero Health and Hailie have launched updated devices equipped with AI-based reminder systems and real-time alerts for caregivers. These tools have become commonplace as of 2025 in pediatric and geriatric asthma care programs. The technology curtails healthcare spending by reducing preventable exacerbations.
Remote Patient Monitoring (RPM) Platforms: These platforms enable healthcare professionals to monitor vital patient data remotely, including spirometry, oxygen saturation, and adherence to medications. Between 2023 and 2024, CMS expanded coverage for RPM under CPT codes 99453 and 99454 for both device provision and monitoring. In 2024, the AMA eliminated the 16-day transmission requirement, broadening access and boosting patient enrollment. In the U.S., Mount Sinai Health System incorporated RPM into asthma care pathways and reported lower high-risk readmission rates. More than 30 state Medicaid programs began reimbursing RPM in 2025, accelerating adoption in community health clinics. RPM tools are now equipped with AI-powered dashboards that predict the occurrence of asthma attacks several minutes—or hours—before symptoms manifest. These platforms turn the management of asthma into a data-driven, proactive process.
The asthma treatment market is segmented into several key regions: North America, Europe, Asia-Pacific, and LAMEA (Latin America, Middle East, and Africa). Here’s an in-depth look at each region:
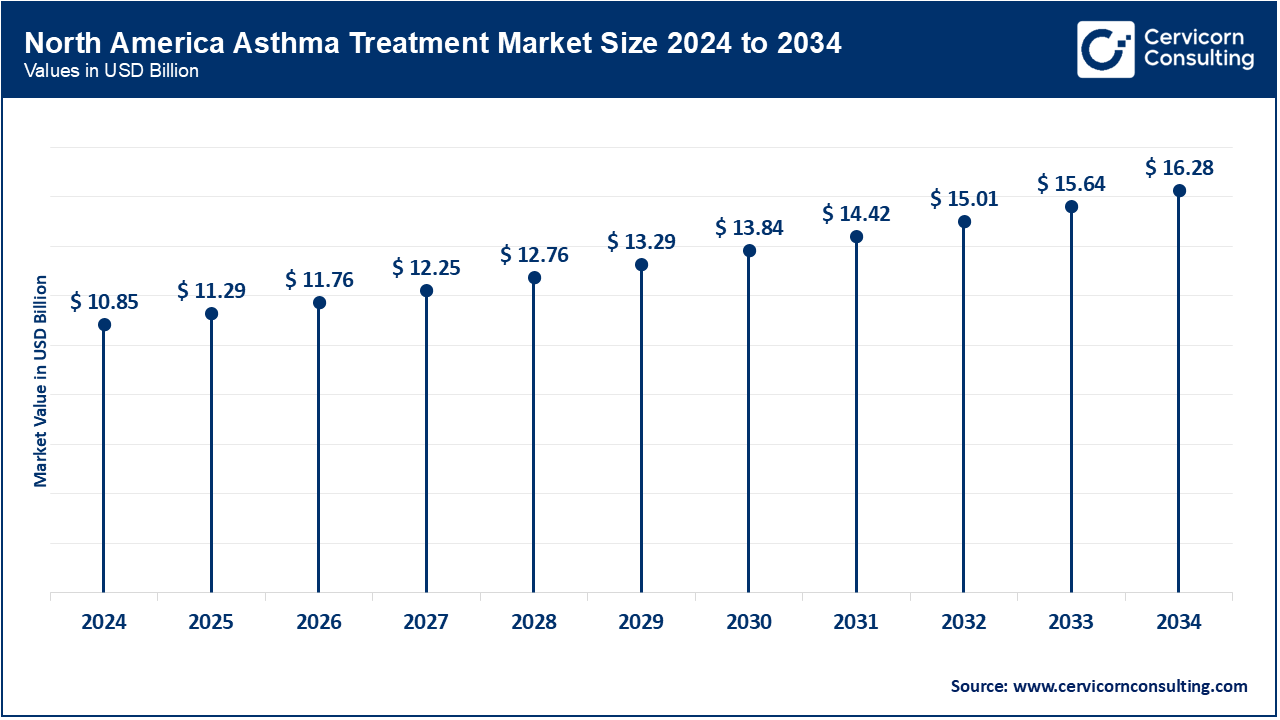
North America maintains its position as a global leader in asthma management because of the newer biologics and inhalers accessible to patients. The CDC reported approximately 25 million Americans with asthma in 2023, resulting in 1.6 million emergency room visits annually. The self-administered Tezepelumab biologics seemed to have greatly benefited rural Americans by around 2024 regionally controlling their severe asthma. Canada's progress is notable too; some provincial public health insurances have started to cover more recent therapies. After the 2023 public campaigns, Mexico's Ministry of Health has also expanded issuing inhaled corticosteroids to rural clinics. Throughout the region, digital monitoring of patients and air quality alert systems are being integrated with clinical guidance. These programs are redefining the personalization and access paradigm for asthma management.
Addressing sustainability and access to biologics remains a focus for Europe’s asthma strategy. The NHS England launched a respiratory program in the UK which favored prescribing ICS inhalers instead of SABA inhalers due to their positive outcomes and for SABA's environmental impact. Germany set out a goal to improve medication adherence by 2024 by expanding reimbursement policies to include smart inhalers and mobile health apps. France's national guideline for asthma updated by late 2024 included monoclonal antibodies for children aged 6 and above with severe asthma. Propellant free inhalers are now granted by all regulatory bodies in Europe which fulfill climate as well as health care goals. Outpatient integration for asthma biologics is now commonplace in hospital settings. Europe is refining the accessibility and precision of asthma care through integrated clinical pathways alongside digital frameworks.
Despite the rapid developments, the Asia-Pacific region still suffers from the disproportionate burden of asthma. Australia's AIHW telemedicine rollout and the funding for biologic therapies came after identifying 2.8 million asthma cases in 2023. In 2024, the National Health Mission of India facilitated access to ICS/LABA through procurement in the rural public sector. China diagnosed a rise in urban asthma cases which led to the implementation of diagnostic phenotypic therapeutic approaches in public hospitals in 2025. Japan revised treatment guidelines with an emphasis on biologic prescription for eosinophilic asthma. South Korea rolled out nation-wide spirometry screening for early diagnosis as of 2023. The region is now shifting from reactive treatment approaches to predictive, comprehensive-data care models alongside widened inhaler provision.
Asthma Treatment Market Revenue Share, By Region, 2024 (%)
| Region | Revenue Share, 2024 (%) |
| North America | 39.70% |
| Europe | 19.90% |
| Asia-Pacific | 26.80% |
| LAMEA | 13.60% |
The LAMEA regions are now addressing a chronic lack of treatment with systematic changes. Brazil's Health Ministry ICSs released clinical protocols and subsidized ICSs after recognizing approximately 20,000 asthma-related deaths over the last decade spanning from 2020 to 2030. Saudi Arabia and UAE in 2023 sponsored access to combination inhalers and issued standardized asthma management protocols. South Africa started providing ICS and long-acting bronchodilator inhalers through its rural clinics in 2024 as part of its essential medicines policy. LAMEA regulatory authorities approved dupilumab and tezepelumab between 2023 and 2025. Train the Trainer campaigns for general practitioners are being implemented in parallel to the national awareness campaigns. There is emerging systematic emphasis on equitable asthma care.
Recent partnerships in the asthma treatment market highlight innovation in drug delivery, biologics, and digital integration. AstraZeneca and Amgen continue their alliance on Tezspire, focusing on expanding global access and home-use options approved in 2024. Teva Pharmaceuticals partnered with MedinCell in 2023 to co-develop long-acting injectables for respiratory care. Boehringer Ingelheim collaborates with Click Therapeutics to develop digital therapeutics for asthma symptom tracking. GSK has teamed up with Propeller Health to integrate smart inhaler technology with patient adherence platforms. These collaborations enhance personalized care, improve adherence, and expand biologic accessibility. Together, they drive the market toward smarter, patient-centered asthma solutions.
Market Segmentation
By Treatment
By Route of Administration
By Patient Type
By Technology Integration
By Distribution Channel
By Region
Chapter 1. Market Introduction and Overview
1.1 Market Definition and Scope
1.1.1 Overview of Asthma Treatment
1.1.2 Scope of the Study
1.1.3 Research Timeframe
1.2 Research Methodology and Approach
1.2.1 Methodology Overview
1.2.2 Data Sources and Validation
1.2.3 Key Assumptions and Limitations
Chapter 2. Executive Summary
2.1 Market Highlights and Snapshot
2.2 Key Insights by Segments
2.2.1 By Treatment Overview
2.2.2 By Route of Administration Overview
2.2.3 By Patient Type Overview
2.2.4 By Technology Integration Overview
2.2.5 By Distribution Channel Overview
2.3 Competitive Overview
Chapter 3. Global Impact Analysis
3.1 Russia-Ukraine Conflict: Global Market Implications
3.2 Regulatory and Policy Changes Impacting Global Markets
Chapter 4. Market Dynamics and Trends
4.1 Market Dynamics
4.1.1 Market Drivers
4.1.1.1 Increased Incidence of Asthma Due to Urbanization and Lifestyle Changes
4.1.1.2 Need for Biologics and Controllers for Severe and Uncontrolled Asthma
4.1.1.3 Initiatives Focused on Screening as well as Early Clinical and Pharmaceutical Action
4.1.2 Market Restraints
4.1.2.1 Prohibitive Pricing of Inhalers and Biologic Therapies
4.1.2.2 Lack of Access to Asthma Care Diagnostics in Low Middle Income Countries
4.1.2.3 Complications and Side Effects of Steroid Use
4.1.3 Market Challenges
4.1.3.1 Incorporation of Smart Devices Data into Clinical Workflows
4.1.3.2 The Algorithms of Asthma Care as An Example of Ethical AI
4.1.3.3 Remote Biologic Delivery
4.1.4 Market Opportunities
4.1.4.1 Collaborations Between Biotech Firms and Tech Companies
4.1.4.2 Digital Services in Respiratory Health
4.1.4.3 Advances in biologics asthma therapy focusing on specific subtypes
4.2 Market Trends
Chapter 5. Premium Insights and Analysis
5.1 Global Asthma Treatment Market Dynamics, Impact Analysis
5.2 Porter’s Five Forces Analysis
5.2.1 Bargaining Power of Suppliers
5.2.2 Bargaining Power of Buyers
5.2.3 Threat of Substitute Products
5.2.4 Rivalry among Existing Firms
5.2.5 Threat of New Entrants
5.3 PESTEL Analysis
5.4 Value Chain Analysis
5.5 Product Pricing Analysis
5.6 Vendor Landscape
5.6.1 List of Buyers
5.6.2 List of Suppliers
Chapter 6. Asthma Treatment Market, By Treatment
6.1 Global Asthma Treatment Market Snapshot, By Treatment
6.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
6.1.1.1 Long-Term Control Medications
6.1.1.2 Quick-Relief Medications
Chapter 7. Asthma Treatment Market, By Route of Administration
7.1 Global Asthma Treatment Market Snapshot, By Route of Administration
7.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
7.1.1.1 Injectable
7.1.1.2 Oral
7.1.1.3 Inhaled
Chapter 8. Asthma Treatment Market, By Patient Type
8.1 Global Asthma Treatment Market Snapshot, By Patient Type
8.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
8.1.1.1 Pediatric Patients (0–17 years)
8.1.1.2 Adult Patients (18–64 years)
8.1.1.3 Geriatric Patients (65+ years)
Chapter 9. Asthma Treatment Market, By Technology Integration
9.1 Global Asthma Treatment Market Snapshot, By Technology Integration
9.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
9.1.1.1 Smart Inhalers (with Bluetooth/AI)
9.1.1.2 Connected Nebulizers
9.1.1.3 Digital Adherence Monitors
9.1.1.4 Remote Patient Monitoring Platforms
Chapter 10. Asthma Treatment Market, By Distribution Channel
10.1 Global Asthma Treatment Market Snapshot, By Distribution Channel
10.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
10.1.1.1 Hospital Pharmacies
10.1.1.2 Retail Pharmacies & Drug Stores
10.1.1.3 Online Pharmacies
Chapter 11. Asthma Treatment Market, By Region
11.1 Overview
11.2 Asthma Treatment Market Revenue Share, By Region 2024 (%)
11.3 Global Asthma Treatment Market, By Region
11.3.1 Market Size and Forecast
11.4 North America
11.4.1 North America Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.4.2 Market Size and Forecast
11.4.3 North America Asthma Treatment Market, By Country
11.4.4 U.S.
11.4.4.1 U.S. Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.4.4.2 Market Size and Forecast
11.4.4.3 U.S. Market Segmental Analysis
11.4.5 Canada
11.4.5.1 Canada Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.4.5.2 Market Size and Forecast
11.4.5.3 Canada Market Segmental Analysis
11.4.6 Mexico
11.4.6.1 Mexico Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.4.6.2 Market Size and Forecast
11.4.6.3 Mexico Market Segmental Analysis
11.5 Europe
11.5.1 Europe Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.5.2 Market Size and Forecast
11.5.3 Europe Asthma Treatment Market, By Country
11.5.4 UK
11.5.4.1 UK Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.5.4.2 Market Size and Forecast
11.5.4.3 UKMarket Segmental Analysis
11.5.5 France
11.5.5.1 France Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.5.5.2 Market Size and Forecast
11.5.5.3 FranceMarket Segmental Analysis
11.5.6 Germany
11.5.6.1 Germany Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.5.6.2 Market Size and Forecast
11.5.6.3 GermanyMarket Segmental Analysis
11.5.7 Rest of Europe
11.5.7.1 Rest of Europe Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.5.7.2 Market Size and Forecast
11.5.7.3 Rest of EuropeMarket Segmental Analysis
11.6 Asia Pacific
11.6.1 Asia Pacific Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.6.2 Market Size and Forecast
11.6.3 Asia Pacific Asthma Treatment Market, By Country
11.6.4 China
11.6.4.1 China Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.6.4.2 Market Size and Forecast
11.6.4.3 ChinaMarket Segmental Analysis
11.6.5 Japan
11.6.5.1 Japan Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.6.5.2 Market Size and Forecast
11.6.5.3 JapanMarket Segmental Analysis
11.6.6 India
11.6.6.1 India Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.6.6.2 Market Size and Forecast
11.6.6.3 IndiaMarket Segmental Analysis
11.6.7 Australia
11.6.7.1 Australia Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.6.7.2 Market Size and Forecast
11.6.7.3 AustraliaMarket Segmental Analysis
11.6.8 Rest of Asia Pacific
11.6.8.1 Rest of Asia Pacific Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.6.8.2 Market Size and Forecast
11.6.8.3 Rest of Asia PacificMarket Segmental Analysis
11.7 LAMEA
11.7.1 LAMEA Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.7.2 Market Size and Forecast
11.7.3 LAMEA Asthma Treatment Market, By Country
11.7.4 GCC
11.7.4.1 GCC Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.7.4.2 Market Size and Forecast
11.7.4.3 GCCMarket Segmental Analysis
11.7.5 Africa
11.7.5.1 Africa Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.7.5.2 Market Size and Forecast
11.7.5.3 AfricaMarket Segmental Analysis
11.7.6 Brazil
11.7.6.1 Brazil Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.7.6.2 Market Size and Forecast
11.7.6.3 BrazilMarket Segmental Analysis
11.7.7 Rest of LAMEA
11.7.7.1 Rest of LAMEA Asthma Treatment Market Revenue, 2022-2034 ($Billion)
11.7.7.2 Market Size and Forecast
11.7.7.3 Rest of LAMEAMarket Segmental Analysis
Chapter 12. Competitive Landscape
12.1 Competitor Strategic Analysis
12.1.1 Top Player Positioning/Market Share Analysis
12.1.2 Top Winning Strategies, By Company, 2022-2024
12.1.3 Competitive Analysis By Revenue, 2022-2024
12.2 Recent Developments by the Market Contributors (2024)
Chapter 13. Company Profiles
13.1 AstraZeneca
13.1.1 Company Snapshot
13.1.2 Company and Business Overview
13.1.3 Financial KPIs
13.1.4 Product/Service Portfolio
13.1.5 Strategic Growth
13.1.6 Global Footprints
13.1.7 Recent Development
13.1.8 SWOT Analysis
13.2 Teva Pharmaceutical Industries Ltd.
13.3 GlaxoSmithKline plc
13.4 Boehringer Ingelheim International GmbH
13.5 Roche Holding AG
13.6 Novartis AG
13.7 Merck & Co., Inc.
13.8 Koninklijke Philips N.V.
13.9 Sanofi-Aventis SA
13.10 MundiPharma